Patient with thrombotic microangiopathy post bone marrow transplant: case study.
A 65-year-old woman, who underwent a bone marrow transplant 10 months ago, presents to the hospital with graft-versus-host disease affecting her skin. She is given immunosuppressive medication and is released.
Two weeks later, she is noted to have elevated blood pressure, 220/115 mm Hg, and headaches. The urinalysis revealed blood and protein.
Her medications include: losartan, meloxicam, prednisone, tacrolimus, and acyclovir.
The patient is readmitted to the hospital.
Labs show elevated creatinine levels. The patient is anemic with a low hemoglobin. The platelet count is low at 52,000/µL. LDH is up. Haptoglobin is down. The urinalysis shows protein and a few red blood cells. Peripheral smear has schistocytes.
A kidney biopsy is performed. The pathologist calls with a diagnosis of thrombotic microangiopathy post bone marrow transplant.
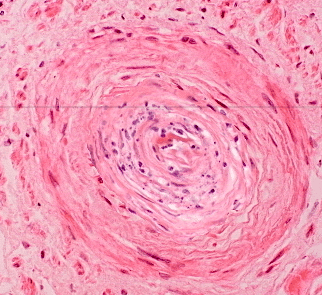
Onion-skinning on kidney biopsy
Patient with thrombotic microangiopathy post bone marrow transplant.
Please answer the following questions:
TMA presents with microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and organ dysfunction. When the kidneys are affected, patients may experience acute kidney injury (AKI), proteinuria, and hypertension. Hematuria can also be present in TMA.
TMA is a serious complication post-transplant, often linked to endothelial injury. The combination of MAHA and thrombocytopenia can lead to significant morbidity. Kidney involvement is particularly concerning, as it can escalate to chronic kidney disease if not managed promptly. Early recognition is crucial for improving outcomes.
Diagnosis is definitively made by kidney biopsy, although thrombocytopenia may prevent this procedure.
Definitive diagnosis through kidney biopsy allows for direct examination of renal tissue, revealing characteristic changes associated with TMA. However, thrombocytopenia poses a risk during the procedure, highlighting the need for careful assessment of patient safety and alternative diagnostic methods when necessary. Sometimes platelets are given to allow for the procedure.
Light microscopy findings consistent with TMA include microthrombi within the glomerular capillary lumens, trapping of fragmented red blood cells (RBCs), and vascular congestion.
Light microscopy in TMA reveals critical changes in the glomeruli, indicating microvascular injury. The presence of microthrombi disrupts normal blood flow, while trapped fragmented RBCs suggest hemolysis. Vascular congestion reflects impaired circulation, highlighting the severity of the underlying pathology.
Electron microscopy shows cellular interposition of new basement membrane or ‘double contouring’ of the glomerular basement membrane (GBM).
In TMA, the glomerular basement membrane exhibits distinctive structural changes, which can be crucial for diagnosis. The ‘double contouring’ reflects abnormal deposition and remodeling, indicating underlying pathological processes. These findings can help differentiate TMA from other renal conditions, guiding treatment strategies.
Laboratory findings include anemia, increased lactate dehydrogenase, decreased haptoglobin levels, and the presence of schistocytes on peripheral blood smear. A direct Coombs test should be negative.
Thrombotic microangiopathy (TMA) often presents with hemolytic anemia and microangiopathic changes. Elevated lactate dehydrogenase indicates tissue damage, while decreased haptoglobin reflects hemolysis. Schistocytes are fragmented red blood cells, signaling mechanical destruction, and a negative Coombs test rules out autoimmune hemolysis.
The reported incidence of thrombotic microangiopathy post bone marrow transplantation is as high as 63%.
Thrombotic microangiopathy (TMA) is a serious complication post-stem cell transplantation, often linked to factors like conditioning regimens, graft-versus-host disease, and immunosuppressive therapies. Its high incidence underscores the need for vigilant monitoring and early intervention to mitigate risks and improve patient outcomes.
The most common types of TMA seen after stem cell transplantation include thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS).
The inciting factor for thrombotic microangiopathy post bone marrow transplantation is thought to be endothelial damage, which may be related to engraftment or a combination of factors such as calcineurin inhibitor (CNI) exposure, total body irradiation, and graft-versus-host disease (GVHD).
Endothelial damage plays a crucial role in the development of thrombotic microangiopathy (TMA) post-stem cell transplantation. This damage can stem from various stressors during the transplant process, including immune responses and toxicities from treatments, which can compromise vascular integrity and lead to complications.
Because TMA is a nonspecific pathologic pattern, careful attention must be paid to the patient’s history, laboratory data, and physical examination to diagnose the underlying etiology.
Careful evaluation is crucial in diagnosing TMA due to its varied presentations and overlapping symptoms with other conditions. A thorough assessment helps identify the specific cause, guiding appropriate treatment. Understanding the patient’s history and lab results can reveal critical clues that influence management strategies.
Learn More:
Tumor Lysis Syndrome Prevention, Test With Solution by Michael Aaronson, Lincoln Nephrology and Hypertension
Using The Number Needed To…
Best Way To Prevent Contrast…
Go to the Top


